Integrated Computational Materials Engineering (ICME)
ICME Overview of the Chemo-mechanical Effects on Magnesium Alloys
Background
To accurately model both chemical and mechanical mechanism effects, one must use multiscale modeling techniques. By starting with the end in mind, the ability to use different forms of magnesium alloys for structural automobile and/or aerospace applications would be the end goal. As an example, the Chevrolet Corvette engine cradle has been cast from an AE44 (Al-Rare Earth Elements ternary) magnesium alloy. The success of the engine cradle project shows that there is opportunity to create lightweight structural members for automobiles, but being able to accurately model the multiscale effects through up scaling and down scaling principles will be vital to further improvement.
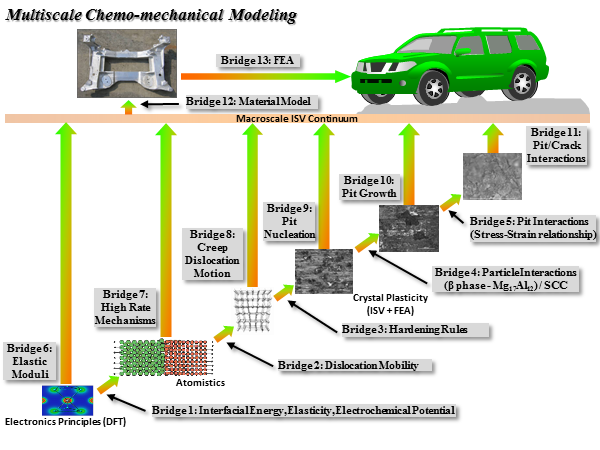
Figure 1. Multiscale modeling diagram example for the chemical-mechanical effects of a magnesium alloy. The hierarchical methodology illustrates the different length scale analyses and various bridges needed.
The importance of figuring out what is needed (down scaling) and what is to be given (up scaling) between length scales creates “bridges” that connect them to construct the entire model. Integrating corrosion mechanisms and recognizing the interaction and effects at all lengths scales becomes most important. The major length scales should all be examined including: electronics scale, nanoscale, microscale, mesoscale, macroscale, and structural scale. Since this modeling philosophy is a top-down approach, the last bridge would be the structural finite element analysis to determine the feasibility of the material for the application. The finite element analysis bridge is proceeded by the material modeling bridge from the macroscale internal state variable (ISV) continuum level. The macroscale ISV continuum level requires information from all length scales (down scaling) to address the performance and manufacturing processes. Each length scale also depends on each other to carry out the history of damage for the material.
Corrosion Mechanisms
The general, pitting, and intergranular corrosion mechanisms must also be studied to better understand how each affect the performance of the alloys. These mechanisms have been previously quantitatively studied for the AZ31 [1], AZ61[2], and AE44 [3] magnesium alloys. General corrosion refers to corrosion dominated by uniform thinning that proceeds without appreciable localized attack. Pitting corrosion is a form of localized attack at relatively small locations compared to the overall exposed surface. Lastly, intergranular corrosion takes place when the corrosion rate of the grain-boundary areas of an alloy exceeds that of the grain interiors [4].
Stress Corrosion Cracking
A considerable body of research outlining the phenomenology of stress corrosion cracking (SCC), especially transgranular stress corrosion (TGSCC) cracking, in magnesium alloys has been done recently [5]. Intergranular stress corrosion cracking (IGSCC) is another important research field that needs to be studied more to enable a better understanding of the full effects of corrosion on magnesium alloys. IGSCC is typically caused by a continuous second phase along grain boundaries. The second phase (β - Mg17Al12) causes microgalvanic corrosion of the adjacent Mg matrix. This understanding is urgently needed because Mg alloys are being increasingly used in load bearing applications [6].
Atomistics
At the atomistic level, mobility effects due to hydrogen embrittlement can be studied. Molecular dynamics (MD) simulations ran by M. Chandler et al. [7] demonstrate that hydrogen causes a reduction of strength and ductility within the bulk material of a face-centered cubic (FCC) system. Although magnesium is not a FCC material, the relationship can be related to other published research. Experimental work completed by M.B. Kannan and W. Dietzel [8], illustrate that both percent elongation to failure and ultimate tensile strength decrease in an AZ80 magnesium alloy once exposed to hydrogen enhanced environments (distilled water and 0.5 wt.% NaCl).
Electronics Principles
At the electronics scale, limited research has been completed using density functional theory (DFT) for magnesium alloy systems. A DFT study of magnesium doped with Al, Ca, and Zn was performed by M.S. Baek et al. [9] gives a good example of how energies and strength of the material can be found.
References
1. C.A. Walton, H.J. Martin, M.F. Horstemeyer, P.T. Wang., Quantification of Corrosion Mechanisms Under Immersion and Salt-Spray Environments on an Extruded AZ31 Magnesium Alloy. Corrosion Science, 56:194-208,(2012)
2. H. J. Martin, M. F. Horstemeyer, and P. T. Wang, Structure–property quantification of corrosion pitting under immersion and salt-spray environments on an extruded AZ61 magnesium alloy, Corrosion Science, 53(4):1348–1361, (2011).
3. H. J. Martin, M. F. Horstemeyer, and P. T. Wang, Comparison of corrosion pitting under immersion and salt-spray environments on an as-cast AE44 magnesium alloy, Corrosion Science, 52(11):3624–3638, (2010).
4. ASM Handbook, Corrosion, vol. 13A, ninth ed., ASM International Handbook Committee, Metals Park, 2003.
5. N. Winzer et al., Characterisation of stress corrosion cracking (SCC) of Mg-Al alloys. Materials Science and Engineering A, 488:339-351, (2008).
6. A. Atrens, N. Winzer, and W. Dietzel. Stress Corrosion Cracking of Magnesium Alloys. Advanced Engineering Materials, 13(1-2):11-18, (2011).
7. M. Chandler et. al, Hydrogen effects on nanovoid nucleation in face-centered cubic single-crystals. Acta Materialia, 56:95-104, (2008).
8. M. B. Kannan and W. Dietzel. Pitting-induced hydrogen embrittlement of magnesium-aluminium alloy. Materials and Design, 42:321-326, (2012).
9. M.S. Baek, D.H. Won, and B.I. Kim. A study on the prediction of the material properties of magnesium alloys using density functional theory method. Korean Journal of Materials Research, 17(12):637-641, (2007).
